|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CLINICAL PRACTICE GUIDELINE |
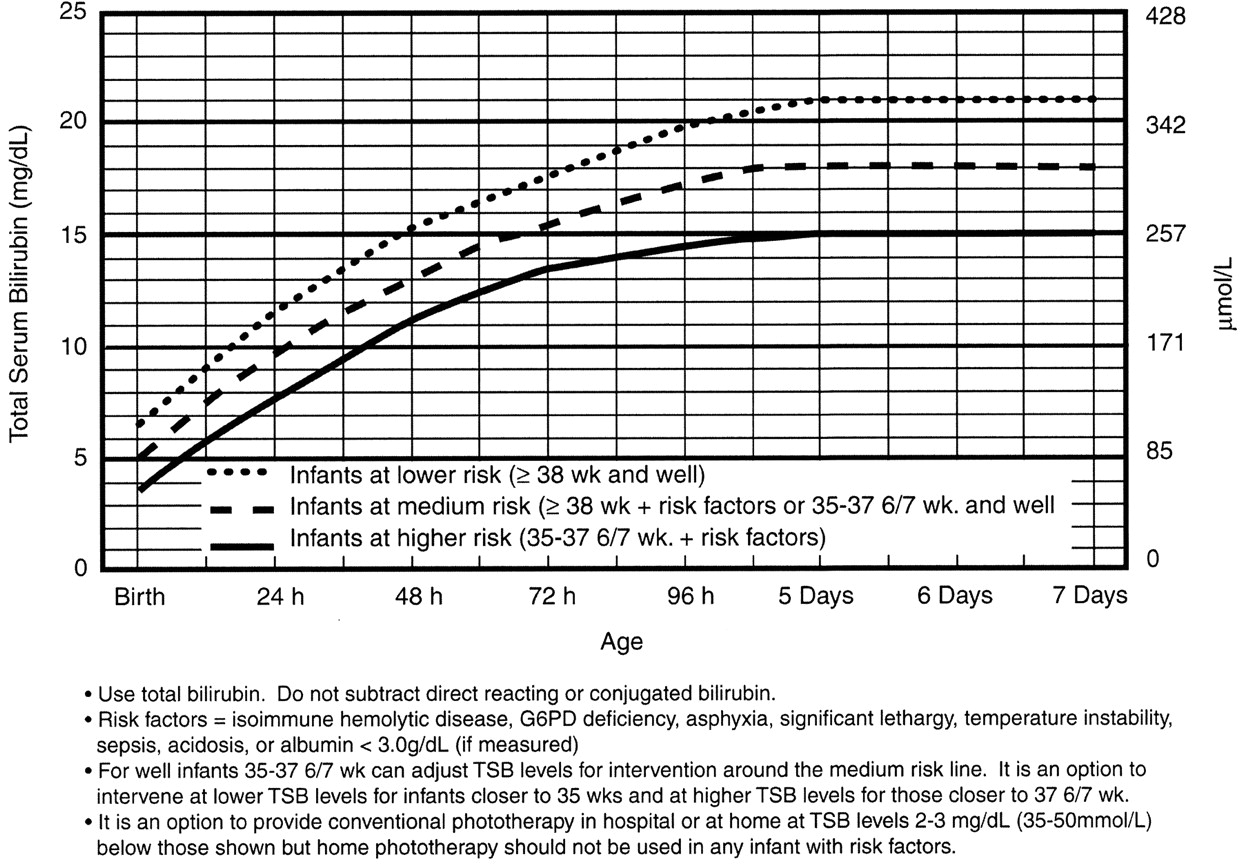
Note: These guidelines are based on limited evidence and the levels shown are approximations. The guidelines refer to the use of intensive phototherapy which should be used when the TSB exceeds the line indicated for each category. Infants are designated as "higher risk" because of the potential negative effects of the conditions listed on albumin binding of bilirubin,45–47 the blood-brain barrier,48 and the susceptibility of the brain cells to damage by bilirubin.48
"Intensive phototherapy" implies irradiance in the blue-green spectrum (wavelengths of approximately 430–490 nm) of at least 30 µW/cm2 per nm (measured at the infant’s skin directly below the center of the phototherapy unit) and delivered to as much of the infant’s surface area as possible. Note that irradiance measured below the center of the light source is much greater than that measured at the periphery. Measurements should be made with a radiometer specified by the manufacturer of the phototherapy system.
See Appendix 2 for additional information on measuring the dose of phototherapy, a description of intensive phototherapy, and of light sources used.
If total serum bilirubin levels approach or exceed the exchange transfusion line (Fig 4), the sides of the bassinet, incubator, or warmer should be lined with aluminum foil or white material.50 This will increase the surface area of the infant exposed and increase the efficacy of phototherapy.51 If the total serum bilirubin does not decrease or continues to rise in an infant who is receiving intensive phototherapy, this strongly suggests the presence of hemolysis.
Infants who receive phototherapy and have an elevated direct-reacting or conjugated bilirubin level (cholestatic jaundice) may develop the bronze-baby syndrome. See Appendix 2 for the use of phototherapy in these infants.
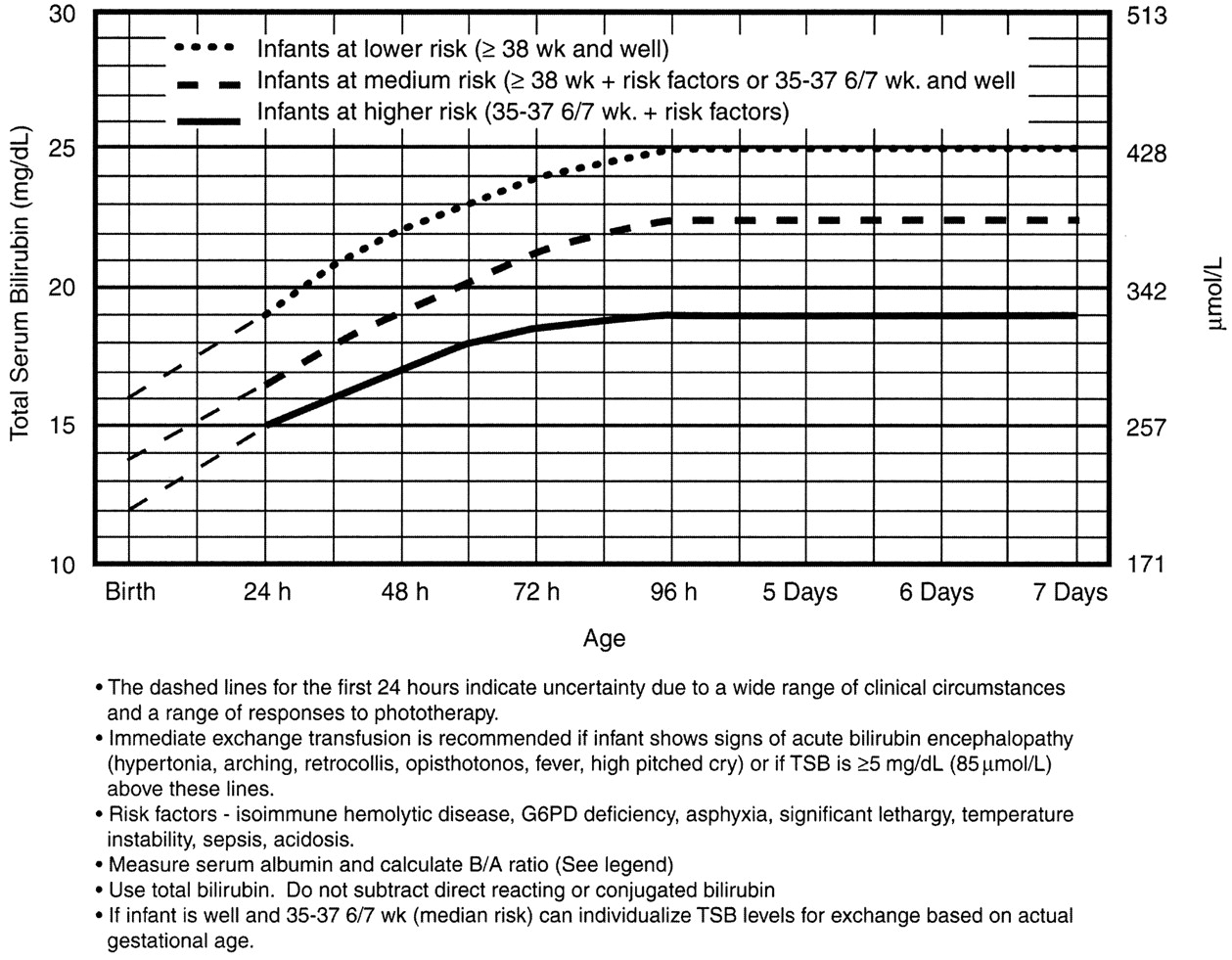
Note that these suggested levels represent a consensus of most of the committee but are based on limited evidence, and the levels shown are approximations. See ref. 3 for risks and complications of exchange transfusion. During birth hospitalization, exchange transfusion is recommended if the TSB rises to these levels despite intensive phototherapy. For readmitted infants, if the TSB level is above the exchange level, repeat TSB measurement every 2 to 3 hours and consider exchange if the TSB remains above the levels indicated after intensive phototherapy for 6 hours.
The following B/A ratios can be used together with but in not in lieu of the
TSB level as an additional factor in determining the need for exchange
transfusion52:
|
|||||||||||||||||||||||||
If the TSB is at or approaching the exchange level, send blood for immediate type and crossmatch. Blood for exchange transfusion is modified whole blood (red cells and plasma) crossmatched against the mother and compatible with the infant.53